Warning after two NHS workers suffer allergic reaction to Covid-19 jab
Health officials said the pair have a ‘significant’ history of allergies.
Regulators have issued a warning that people who have a history of “significant” allergic reactions should not currently receive the Pfizer/BioNTech Covid-19 vaccine after two people who had the jab on Tuesday had allergic reactions.
Two NHS staff members who received the vaccine on the first day of the mass vaccination programme suffered an allergic reaction, the NHS in England has confirmed.
It is understood both have a significant history of allergic reactions – to the extent where they need to carry an adrenaline auto-injector with them.
They were among thousand of people who received the vaccine on Tuesday.
The Medicines and Healthcare products Regulatory Agency (MHRA) has given precautionary advice to NHS trusts that anyone who has a history of “significant” allergic reactions to medicines, food or vaccines should not receive the vaccine.
The NHS in England said all trusts involved with the vaccination programme have been informed.
This means that anyone scheduled to receive the vaccine on Wednesday will be asked about their history of allergic reactions.
Professor Stephen Powis, national medical director for the NHS in England, said: “As is common with new vaccines, the MHRA have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday.
“Both are recovering well.”
The MHRA advice states: “Any person with a history of a significant allergic reaction to a vaccine, medicine or food (such as previous history of anaphylactoid reaction or those who have been advised to carry an adrenaline auto-injector) should not receive the Pfizer/BioNtech vaccine.
“Resuscitation facilities should be available at all times for all vaccinations. Vaccination should only be carried out in facilities where resuscitation measures are available.”
The two NHS workers developed symptoms of “anaphylactoid reaction” shortly after receiving the vaccine, but both recovered after the appropriate treatment.
Pfizer said the vaccine was “well tolerated” during the trials with “no serious safety concerns”.
A spokeswoman said: “We have been advised by MHRA of two yellow card reports that may be associated with allergic reaction due to administration of the Covid-19 BNT162b2 vaccine.
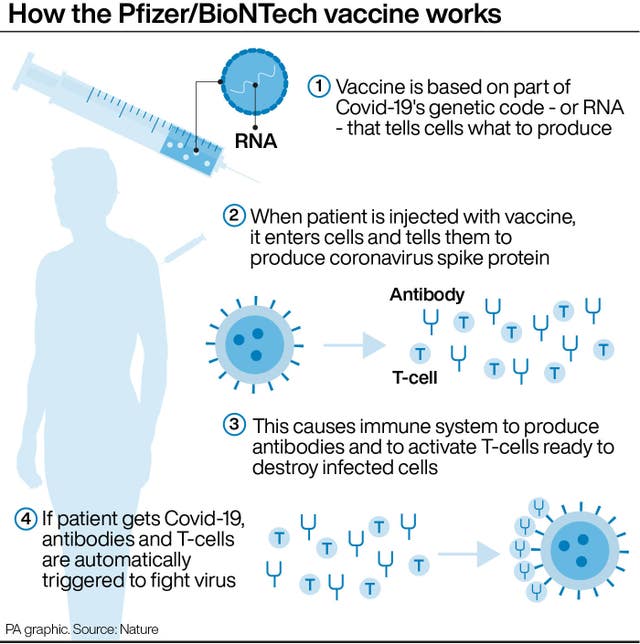
“As a precautionary measure, the MHRA has issued temporary guidance to the NHS while it conducts an investigation in order to fully understand each case and its causes.
“Pfizer and BioNTech are supporting the MHRA in the investigation.
“In the pivotal phase three clinical trial, this vaccine was generally well tolerated with no serious safety concerns reported by the independent Data Monitoring Committee.
“The trial has enrolled over 44,000 participants to date, over 42,000 of whom have received a second vaccination.”
The yellow card scheme is the UK system for collecting and monitoring information on suspected safety concerns or incidents involving medicines and medical devices.
Commenting on the development, Peter Openshaw, past-president of the British Society for Immunology and professor of experimental medicine at Imperial College London, said: “As with all food and medications, there is a very small chance of an allergic reaction to any vaccine.
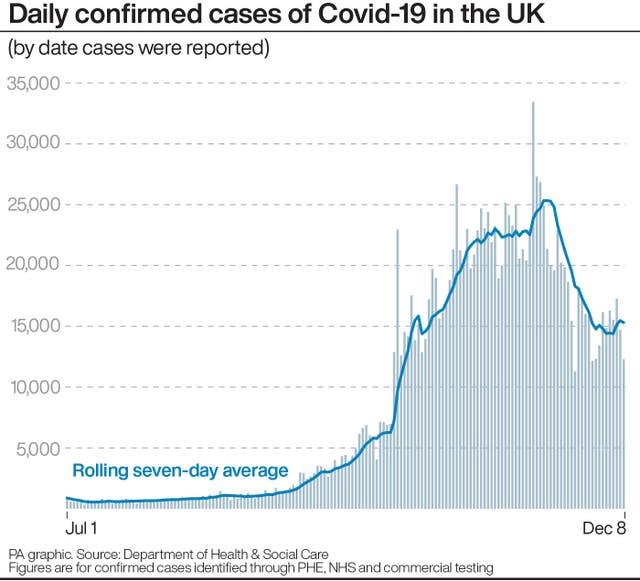
“However, it is important that we put this risk in perspective. The occurrence of any allergic reaction was one of the factors monitored in the phase three clinical trial of this Pfizer/BioNTech Covid-19 vaccine, the detailed data from which was released yesterday.
“In this, they reported a very small number of allergic reactions in both the vaccine and placebo groups (0.63% and 0.51%).
“Similar to the rollout of all new vaccines and medications, this new Covid-19 vaccine is being monitored closely by the MHRA.
“They will now investigate these cases in more detail to understand if the allergic reactions were linked to the vaccine or were incidental.
“The fact that we know so soon about these two allergic reactions and that the regulator has acted on this to issue precautionary advice shows that this monitoring system is working well.”
The patient safety leaflet for the vaccine cautions that anyone with an allergy to any of the active substances in the vaccine should not receive the jab.
It adds: “Signs of an allergic reaction may include itchy skin rash, shortness of breath and swelling of the face or tongue.”





